The Intrafix® Primeline portfolio includes:
§ Infusion devices of type G (Gravity) - for gravity infusion
§ Type P (pressure) infusion devices - for gravity and pressure infusion with compatible (non-dedicated) infusion pumps (pressure resistant up to 2 bar according to ISO 8536-8).
Advantages
Three-part drip chamber with:
§ sharp piercing pin for easy piercing
§ manual ventilation flap with integrated ventilation filter (bacterial and viral filtration efficiency) to prevent microbial contamination when venting the solution container
§ transparent dome with integrated macro dropper (20 drops per 1 ml) for constant visual monitoring of the flow rate of infusion solutions
§ flexible pump chamber for easy and quick adjustment of the liquid level
§ Liquid filter (filter membrane) in the drip chamber bottom:
§ with a pore size of 15 µm to protect against coarse particles
Hose
§ Dimensions: 3 x 4.1 mm; available in different lengths: 150 cm, 180 cm, 230 cm
§ Pressure resistant up to 2 bar and suitable for use with infusion pumps designed for a 3 x 4.1 mm hose
Roller clamp
§ Allows fine and flexible adjustment of flow rate
§ Provides a spike guard for safe disposal
§ With parking device to assist handling during venting
Patient connection
§ With luer lock connection
§ Also available with a check valve to prevent the risk of fluid and blood reflux into the infusion line (concerns ref 4063287, 4062983L)
§ Also available with end cap PrimeStop (Protective cap lined with a hydrophobic, bacteria-proof membrane to prevent the leakage of liquid during venting and to protect against contamination (chemical and bacterial tightness/closed system according to NIOSH 1; 3; 4 )) - (refers to ref 4062982L, 4062983L)
Material related information
§ Not made from natural rubber latex
§ Not made with DEHP
§ Not made of PVC (only for versions with PUR hose, concerns ref 4062191, 0086774R)
§ Not made with BPA (except infusion sets Discofix® C three-way tooth)
§ Useful life
§ The duration of use depends on the intended therapy according to the product information or solution.
§ In general, the replacement of infusion devices should be done according to national recommendations (eg KRINKO recommendations) and/or internal guidelines.
§ IV-delivery pressure sets (P) should be changed at the latest after 24 hours when used with an infusion pump.
Different article configurations
§ Different hose lengths for better patient mobility
§ Tube material PUR (Neutrapur) or UV-protected tube material (UV-Protect) up to 500 nm wavelength
§ Infuvalve® check valve prevents the reflux of fluids and blood
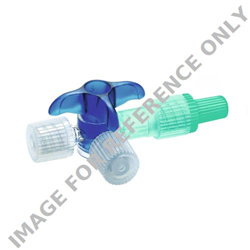
§ Discofix® C three-way valve for needle-free injection, aspiration and parallel infusion
§ Needle-free access to infusion systems with Safeflow or CareSite® connectors to reduce the risk of needlestick injury.





 Documentations
Documentations